Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Fe
Fe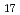 intermetallic compound
intermetallic compoundCao, Y.*; Zhou, H.*; Khmelevskyi, S.*; Lin, K.*; Avdeev, M.*; Wang, C.-W.*; Wang, B.*; Hu, F.*; Kato, Kenichi*; Hattori, Takanori; et al.
Chemistry of Materials, 35(8), p.3249 - 3255, 2023/04
Times Cited Count:1 Percentile:45.8(Chemistry, Physical)Hydrostatic and chemical pressure are efficient stimuli to alter the crystal structure and are commonly used for tuning electronic and magnetic properties in materials science. However, chemical pressure is difficult to quantify and a clear correspondence between these two types of pressure is still lacking. Here, we study intermetallic candidates for a permanent magnet with a negative thermal expansion (NTE). Based on in situ synchrotron X-ray diffraction, negative chemical pressure is revealed in Ho Fe
Fe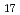 on Al doping and quantitatively evaluated by using temperature and pressure dependence of unit cell volume. A combination of magnetization and neutron diffraction measurements also allowed one to compare the effect of chemical pressure on magnetic ordering with that of hydrostatic pressure. Intriguingly, pressure can be used to control suppression and enhancement of NTE. Electronic structure calculations indicate that pressure affected the top of the majority band with respect to the Fermi level, which has implications for the magnetic stability, which in turn plays a critical role in modulating magnetism and NTE. This work presents a good example of understanding the effect of pressure and utilizing it to control properties of functional materials.
on Al doping and quantitatively evaluated by using temperature and pressure dependence of unit cell volume. A combination of magnetization and neutron diffraction measurements also allowed one to compare the effect of chemical pressure on magnetic ordering with that of hydrostatic pressure. Intriguingly, pressure can be used to control suppression and enhancement of NTE. Electronic structure calculations indicate that pressure affected the top of the majority band with respect to the Fermi level, which has implications for the magnetic stability, which in turn plays a critical role in modulating magnetism and NTE. This work presents a good example of understanding the effect of pressure and utilizing it to control properties of functional materials.
 -LiAl; Relation to the defect structure
-LiAl; Relation to the defect structureSugai, Hiroyuki
Solid State Ionics, 177(39-40), p.3507 - 3512, 2007/01
The diffusion coefficient and its activation energy (116.3  11.7 kJ/mol) of tritium in an intermetallic compound
11.7 kJ/mol) of tritium in an intermetallic compound  -LiAl are determined at temperatures from 700 to 848 K. Though the present result for the diffusion coefficient is almost the same as that reported previously, the present result for the activation energy turns out nearly twice of that (64.9
-LiAl are determined at temperatures from 700 to 848 K. Though the present result for the diffusion coefficient is almost the same as that reported previously, the present result for the activation energy turns out nearly twice of that (64.9  3.8 kJ/mol). The present result for the activation energy is consistent with the systematics that an increase of lithium concentration in Al-Li systems increases the activation energy, but the previous result is not. Furthermore, a consideration of the crystal structure and defect structure suggests that tritium diffuses and is impeded by the attractive interaction with lithium atom at lithium sublattices.
3.8 kJ/mol). The present result for the activation energy is consistent with the systematics that an increase of lithium concentration in Al-Li systems increases the activation energy, but the previous result is not. Furthermore, a consideration of the crystal structure and defect structure suggests that tritium diffuses and is impeded by the attractive interaction with lithium atom at lithium sublattices.
 -LiAl; Relation to the defect structure
-LiAl; Relation to the defect structureSugai, Hiroyuki
Solid State Ionics, 177(39-40), p.3507 - 3512, 2007/01
Times Cited Count:3 Percentile:17.83(Chemistry, Physical)The diffusion coefficients and its activation energy (103.7 9.5 kJ/mol) for tritium in intermetallic compound
9.5 kJ/mol) for tritium in intermetallic compound  -LiAl are determined at temperatures from 699 to 886 K. Though the present result for the diffusion coefficient is almost the same as that reported earlier, the activation energy turns out nearly twice of that (64.9
-LiAl are determined at temperatures from 699 to 886 K. Though the present result for the diffusion coefficient is almost the same as that reported earlier, the activation energy turns out nearly twice of that (64.9 3.8 kJ/mol) reported earlier. On the basis of the crystal structure and defect structure, the large activation energy of this study suggest that tritium diffuses interstitially and is impeded by an attractive interaction with lithium atoms in lithium sublattices.
3.8 kJ/mol) reported earlier. On the basis of the crystal structure and defect structure, the large activation energy of this study suggest that tritium diffuses interstitially and is impeded by an attractive interaction with lithium atoms in lithium sublattices.
Nakajima, Kunihisa; Nakazono, Yoshihisa; Arai, Yasuo
Recent Advances in Actinide Science, p.448 - 450, 2006/06
A pyrochemical process for the metallurgical treatment of the spent nuclear fuels contains the Cd-distillation step, where Pu is recovered as a residue. For understanding this Cd-ditillation behavior the samples of PuCd +PuCd
+PuCd and PuCd
and PuCd +PuCd
+PuCd were prepared and Knudsen-cell mass-spectrometric measurements were carried out to determine the vapour pressures of Cd(g) over these samples. Further,the thermodynamic quantities of PuCd
were prepared and Knudsen-cell mass-spectrometric measurements were carried out to determine the vapour pressures of Cd(g) over these samples. Further,the thermodynamic quantities of PuCd and PuCd
and PuCd were evaluated from these vapour pressures.
were evaluated from these vapour pressures.
 Pb
Pb intermetallic compound
intermetallic compoundOno, Masao; Huang, X. S.*; Shibata, Yasuhiro*; Iguchi, Yusuke*; Sakai, Seiji; Maekawa, Masaki; Chen, Z. Q.*; Osakabe, Toyotaka; Kawasuso, Atsuo; Naramoto, Hiroshi*; et al.
Proceedings of 1st International Conference on Diffusion in Solids and Liquids (DSL 2005), p.531 - 533, 2005/07
Recently, we formed atomic-scale graded structures in some miscible alloys and observed the decomposition in Bi Pb
Pb intermetallic compound by sedimentation of atoms under strong gravitational field. In this study, we measured positron lifetime of centrifuged Bi
intermetallic compound by sedimentation of atoms under strong gravitational field. In this study, we measured positron lifetime of centrifuged Bi Pb
Pb , to which the composition change was very small as it was treated at low temperature. It was found that the positron lifetime became longer than that of starting state. This indicated that the point defects (vacancy or divacancy) increased in the sample by centrifugal treatment. We are now investigating the relationship between increase in point defects and sedimentation of atoms.
, to which the composition change was very small as it was treated at low temperature. It was found that the positron lifetime became longer than that of starting state. This indicated that the point defects (vacancy or divacancy) increased in the sample by centrifugal treatment. We are now investigating the relationship between increase in point defects and sedimentation of atoms.

Nakajima, Kunihisa; Arai, Yasuo; Yamashita, Toshiyuki
Journal of Physics and Chemistry of Solids, 66(2-4), p.639 - 642, 2005/02
Times Cited Count:2 Percentile:12.62(Chemistry, Multidisciplinary)PuCd intermetallic compound was prepared by heating pure Pu and Cd metals at about 950K. PuCd
intermetallic compound was prepared by heating pure Pu and Cd metals at about 950K. PuCd was found to be a prototype of CdI
was found to be a prototype of CdI by means of powder X-ray diffractometry. Mass-spectrometric experiment was performed in the temperature range of 650-770K. It was found that the vapor pressures of Cd over PuCd
by means of powder X-ray diffractometry. Mass-spectrometric experiment was performed in the temperature range of 650-770K. It was found that the vapor pressures of Cd over PuCd +Pu were three to five orders of magnitude lower than those over Cd in this temperature range. From these vapor pressures, Gibbs free energy of formation of PuCd
+Pu were three to five orders of magnitude lower than those over Cd in this temperature range. From these vapor pressures, Gibbs free energy of formation of PuCd was evaluated.
was evaluated.
Ono, Masao; Huang, X.*; Kinoshita, Takahiro*; Ueno, Hideto*; Osakabe, Toyotaka; Mashimo, Tsutomu
Defect and Diffusion Forum, 237-240(2), p.1101 - 1104, 2005/00
Ultra-strong gravitational field can induce sedimentation of even atoms in condensed matter. We had realized sedimentation of substitutional solute atoms in some miscible alloys. How about in the other alloys? So, In this study, the ultra-centrifuge experiments were performed on an intermetallic compound of Bi-Pb system (Bi Pb
Pb ) by changing time duration of experiment time (experimental conditions; maximum centrifugal force: 1.0
) by changing time duration of experiment time (experimental conditions; maximum centrifugal force: 1.0 10
10 g level, temperature: 130-150
g level, temperature: 130-150  C, duration: 30-150h, state: solid). Composition changes were observed in the centrifuged samples. And, it was found that the Bi phase appeared from starting state of Bi
C, duration: 30-150h, state: solid). Composition changes were observed in the centrifuged samples. And, it was found that the Bi phase appeared from starting state of Bi Pb
Pb around the weak gravitational field region of the sample. These results showed that sedimentation of substitutional solute atoms occurred, and induced the structure change in intermetallic compounds.
around the weak gravitational field region of the sample. These results showed that sedimentation of substitutional solute atoms occurred, and induced the structure change in intermetallic compounds.
 melt containing K
melt containing K TaF
TaF ; Electrochemical study
; Electrochemical studyMehmood, M.*; Kawaguchi, Nobuaki*; Maekawa, Hideki*; Sato, Yuzuru*; Yamamura, Tsutomu*; Kawai, Masayoshi*; Kikuchi, Kenji
Materials Transactions, 44(2), p.259 - 267, 2003/02
Times Cited Count:9 Percentile:52.01(Materials Science, Multidisciplinary)Electrochemical study has been carried out on the electro-deposition of tantalum in LiF-NaF-CaF melt containing K
melt containing K TaF
TaF at 700
at 700 C. This has been done for determining the mechanistic features for preparing electrolytic coating of tantalum on nickel and tungsten substrates. Electro-deposition of metallic tantalum occurs primarily by electro-reduction of Ta(V). Pure metallic tantalum without any entrapped salt is successfully deposited on tungsten by galvanostatic polarization at reasonably low current densities. An additional feature on nickel is the formation of an intermetallic compound at potential 0.25V nobler than that of pure tantalum as a result of underpotential deposition of tantalum. This intermetallic compound covers the surface within a short time followed by deposition of pure tantalum, although intermetallic compound keeps growing at the interface of pure tantalum deposit and the substrate as a result of diffusion.
C. This has been done for determining the mechanistic features for preparing electrolytic coating of tantalum on nickel and tungsten substrates. Electro-deposition of metallic tantalum occurs primarily by electro-reduction of Ta(V). Pure metallic tantalum without any entrapped salt is successfully deposited on tungsten by galvanostatic polarization at reasonably low current densities. An additional feature on nickel is the formation of an intermetallic compound at potential 0.25V nobler than that of pure tantalum as a result of underpotential deposition of tantalum. This intermetallic compound covers the surface within a short time followed by deposition of pure tantalum, although intermetallic compound keeps growing at the interface of pure tantalum deposit and the substrate as a result of diffusion.
 compound
compoundAkabori, Mitsuo; R.G.Haire*; J.K.Gibson*; Okamoto, Yoshihiro; Ogawa, Toru
Journal of Alloys and Compounds, 257, p.268 - 272, 1997/00
Times Cited Count:2 Percentile:29.44(Chemistry, Physical)no abstracts in English
Akabori, Mitsuo; R.G.Haire*; J.K.Gibson*; Okamoto, Yoshihiro; Ogawa, Toru
Journal of Nuclear Materials, 247, p.240 - 243, 1997/00
Times Cited Count:2 Percentile:23.01(Materials Science, Multidisciplinary)no abstracts in English
J.K.Gibson*; R.G.Haire*; E.C.Beahm*; M.M.Gensini*; Maeda, Atsushi; Ogawa, Toru
Journal of Nuclear Materials, 211, p.215 - 222, 1994/00
Times Cited Count:9 Percentile:63.31(Materials Science, Multidisciplinary)no abstracts in English
 Pb
Pb
; Nagame, Yuichiro;
J.Less-Common Met., 119, p.211 - 217, 1986/00
Times Cited Count:5 Percentile:64.2(Chemistry, Physical)no abstracts in English

; ; ;
Journal of the Physical Society of Japan, 16(7), p.1486 - 1486, 1961/00
Times Cited Count:20no abstracts in English
Kim, Jae-Hwan; Nakamichi, Masaru
no journal, ,